Additional information
| Active substance | Dabigatran |
|---|---|
| Water Retention | No significant water retention |
| Hepatotoxicity | No significant hepatotoxicity |
| Lab Test | Monitoring of renal function and possibly coagulation parameters |
| Also known as | Dabigatran |
| Blood pressure | Typically does not affect blood pressure |
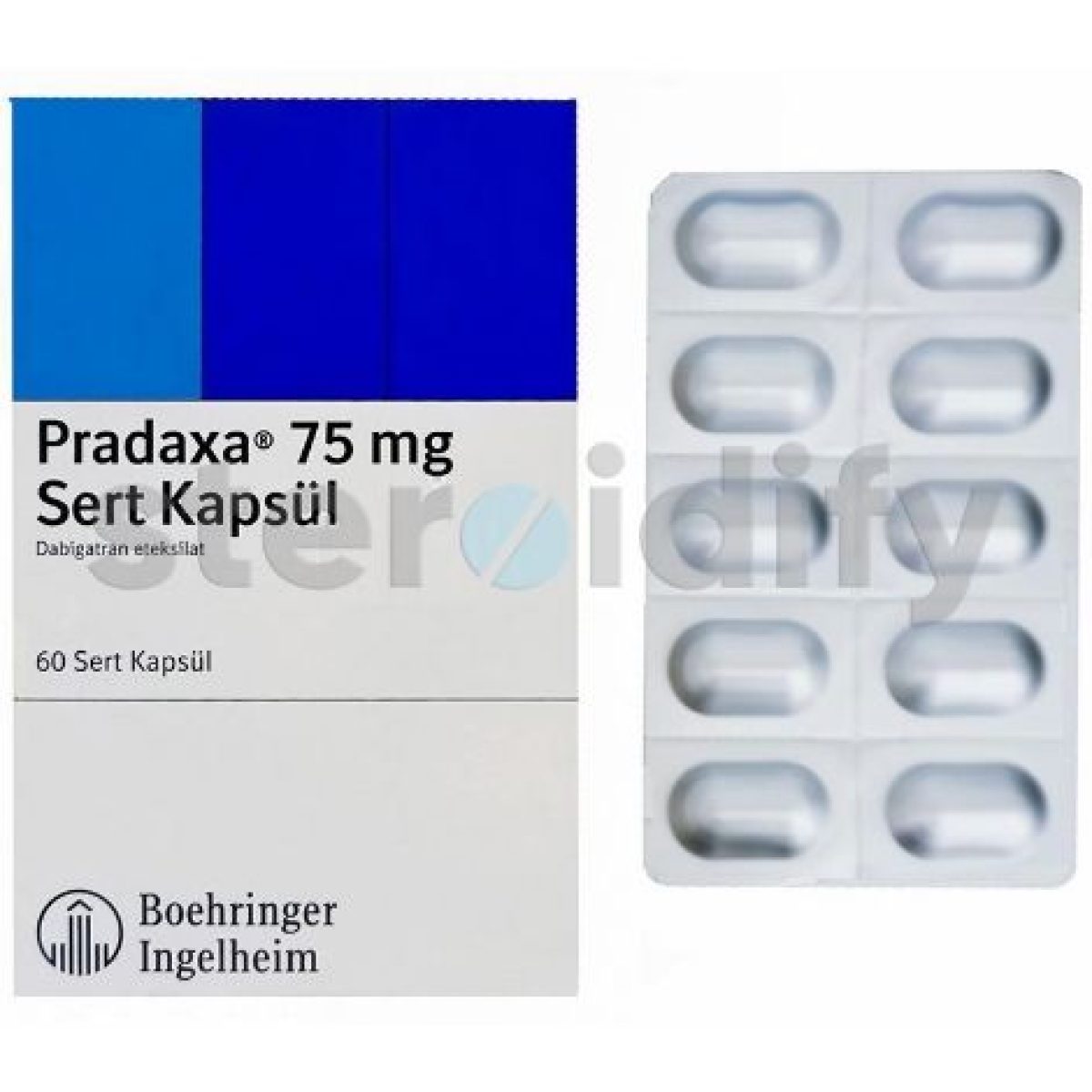
| Trade name | Pradaxa |
| FORM | 60 caps x 75 mg |
| Storage conditions | Store at room temperature away from moisture and heat |
| Chemical name | N-{2-[4-(N'-{[(Hexyloxy)carbonyl]carbamimidoyl}carbamimidoyl)phenylaminomethyl]-1-methyl-1H-benzimidazol-5-ylcarbonyl}-N-pyridin-2-yl-beta-alanine ethyl ester |
| Formula | C34H41N7O5 |
| Substance class | Direct thrombin inhibitor |
| Main action | Anticoagulant |
| Half-life | 12 to 17 hours |
| Dosage (medical) | 150 mg or 110 mg twice daily depending on renal function |
| Dosage (sports) | Not applicable as it is not used for sports enhancement |
| Effects | Reduces the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation |
| Side effects | Bleeding, gastrointestinal upset, heartburn, nausea |
| Use in sports | None, as it does not enhance athletic performance |
| Manufacturer | Boehringer |


Reviews
There are no reviews yet.